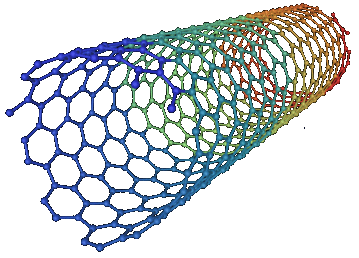
_process section (discharge) for electrical nanotubes
Study of aromatic molecules in nanotubeshaving electronic properties SWCNTs
Researcher and author: Dr. Afshin Rashid
Note: It is known that carbon nanotubes have a strong interaction with aromatic molecules such as graphite surface. SWCNTs can be considered as an extended π-electron system in 4, which can interact with other π-electron systems through interactions π-π relationship
Such π-π interactions act as the main driving force for the adsorption of DNA and aromatic polymers on the nanotube surface. It is interesting and important to discover how selective interactions between π-conjugated compounds and SWCNTs occur. Certain aromatic monomers and polymers can selectively dissolve semiconducting or metallic SWCNTs. π-π bonding between the aromatic molecule and the surface of SWCNTs is done in a selective direction, which can be one of the reasons for the selective performance of these aromatic molecules. Selective non-covalent functionalization of semiconductor SWCNTs was characterized by porphyrin chemistry. Some aromatic molecules can form charge transfer complexes with metallic SWCNTs.
It has been determined with porphyrin chemistry that some aromatic molecules can form charge transfer complexes with metal SWCNTs. Nano Fluorene polymer derivatives can selectively form nanotubes (7,5) and (8, 6) and (10,5) enrichment . Polymer structure Polymer structure and solvent structure both strongly influence and in some cases lead to selectivity are very high in terms of diameter and chiral angle . The molecules of condensation (Diels-Alder) acenes are used to disperse making nanotubes with a large diameter a>performhigher certainty of separation of chiral nanotubes This work makes the selectively right- or left-handed SWCNTs. isolate a i=33>. The degree of chiral selectivity is optimized by controlling the dihedral angle between porphyrins. It is interesting that diporphyrin molecules can be right-handed or left-handed. molecular tweezers to be able to Chiral diporphyrin isomers act as are separated by derivatives (poly-perylene). In addition, by controlling the dispersion-separation process, metal nanotubes and then semiconductor nanotubes can be separated. did while small chiral nanotubes using dense benzoic aromatics Large chiral nanotubes can be separated is concentrated; Meanwhile, the aggressiveness of nanotubes with a larger diameter (~6.1 nm) has been proposed. Semiconductor carbon nanotubes whose diameter is larger (6mn); This type of nanotubes have better efficiency in electronic equipment. selection of nanotubes with a small diameter (<2.1 nm) . A
Conclusion :
The advantage of using aromatic molecules is their structural diversity and their similarity to nanotubes. Molecules can be designed and synthesized to increase the accuracy of separation operations. Generally, there are two important points in the bonding of aromatic molecules and covalent bonding of SWCNTs nanotubes. First, different absorption to be done by means of molecules, and the other one is enhanced scattering of SWCNTS absorption. Therefore, in these non-covalent methods, the separation efficiency is always affected by the solvents.
Researcher and author: Dr. Afshin Rashid
Specialized doctorate in nano-microelectronics