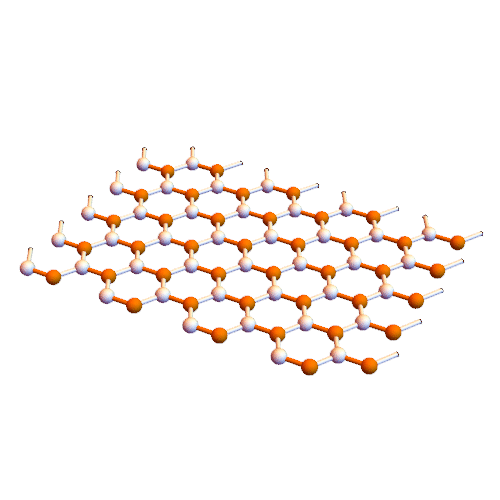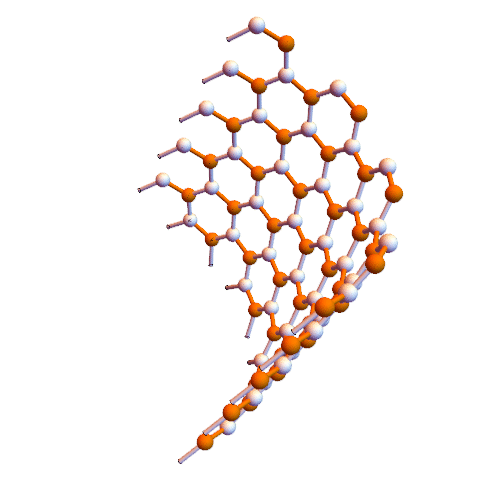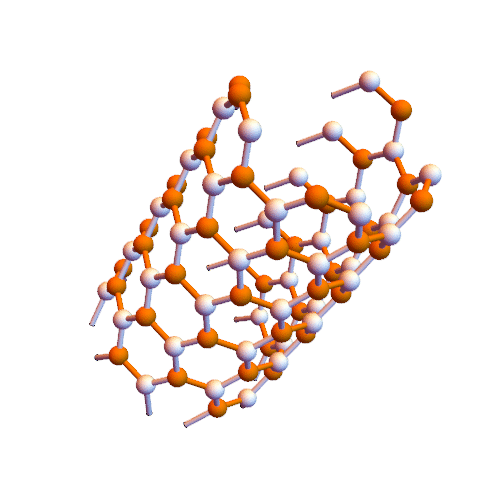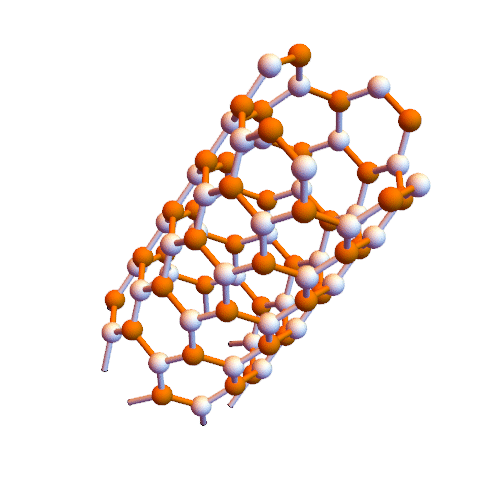
Interaction of aromatic molecules of nanotubes (SWCNTs and SWCNTs) based on nano-microelectronics (PhD)
Researcher and author: Dr. ( Afshin Rashid)
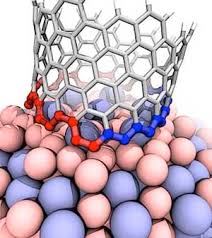
Note: It is clear that carbon nanotubes have strong interactions with aromatic molecules such as graphite surface. SWCNTs can be considered as extended π-electron systems that can interact with other π-electron systems through π-π interactions.
Such π-π interactions act as the primary driving force for the adsorption of DNA and aromatic polymers onto the nanotube surface . It is interesting to discover how the selective interactions between the π conjugates and SWCNTs occur. Certain aromatic monomers and polymers can selectively dissolve semiconductor or metal SWCNTs . The π-π bonding between the aromatic molecule and the surface of the SWCNTs occurs in selective direction, which may be one of the reasons for the selective performance of these aromatic molecules. The selective noncovalent functionalization of semiconductor SWCNTs is characterized by porphyrin chemistry. Some aromatic molecules can be complexed The charge transfer with metal SWCNTs form. Fluorene polymer derivatives can be selectively arranged in both polymer and solvent structures, respectively, and in some cases lead to very high selectivity in terms of chiral diameter and angle . The Dales-Alder compression molecules (acenes) were used to disperse high-diameter nanotubes. The focus was on the selection of aromatic molecules of low-diameter nanotubes (<2.1 nm), while the selection of aromatic molecules was applied to the structure of higher diameter nanotubes (~ 6.1 nm).
Semiconductor carbon nanotubes with larger diameter have better efficiency in electronic equipment. Selective dispersion of SWCNTs using aromatics Dense benzoids have been propagated such as pentacene, anthracene and quaterylene. Large chiral nanotubes can be separated using dense benzoid aromatics while small chiral nanotubes are separated by polypropylene derivatives. In addition, by controlling the dispersion-separation process, metal nanotubes and then semiconductor nanotubes can be separated. Selectively isolate right or left SWCNTs. Chiral selectivity is optimized by two-way angle control between porphyrins. Interestingly, the diporphyrin molecules can be either right-handed or left-handed Prior to this, electron scattering by detecting the error of the carbon network in the space of carbon nanotubes SWCNTs and SWCNTs. Or they can partially separate SWCNT and SWCNTs enantiomers. This enables the aromatic nano-molecules to be more reliably separated by chiral nanotubes.
Conclusion :
The advantage of using aromatic molecules is their structural diversity and their similarity to nanotubes. Molecules can be designed and synthesized to increase the accuracy of separation operations and to make the separation unrealistic . There are generally two types of covalent bonds: first, the different adsorption by molecules and the other is the enhanced scattering of SWCNTS . Therefore in these non-quantitative methods, the separation efficiency is always affected by the solvents .
Researcher and author: Dr. ( Afshin Rashid)