_Part of the process (discharging) for electric nanotubes
Electrical strength and elasticity (Elasticity) about 1 TeraPascal in the structure of multi-layer carbon nanotubes CNTs _(important article, pay more attention)
Author and researcher : Dr. (Afshin Rashid)
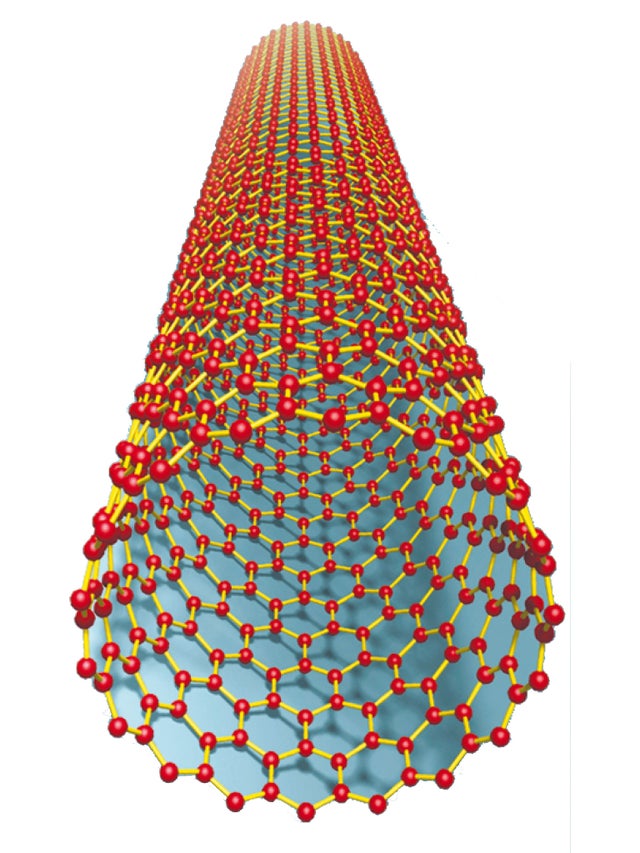
Note: The carbon atoms of a graphitic sheet (graphene) form a planar lattice network, where each atom is connected to three neighboring atoms through a strong chemical bond. Because of these strong bonds, the elastic modulus of graphite base is one of the largest known materials.
For this reason, CNTs are final fibers with high strength. SWNTs are harder than steel and are highly resistant to damage from physical forces. Pressing on the tip of the nanotube causes it to bend, but without damaging the tip. When the force is removed, the tip returns to its original position.
This feature makes CNTs very useful as probe guides for high-resolution scanning probe microscopy. Quantifying these effects has been difficult, and an exact numerical value has not been agreed upon. The free ends of a free nanotube can be pulled out of their equilibrium position and the force required to compress the nanotube can be measured. The current modulus value of SWNTs is about 1 TeraPascal.
In the structure of multi-layered carbon nanotubes, CNTs, there is a unique strength and an unparalleled ability to achieve excellent electrical properties of semiconductors or metallic nanotubes. The special nature of carbon in conductive nanotubes is combined with the molecular deficiency of single-walled CNTs to provide them with exceptional material properties such as very high electrical and thermal conductivity in the structure of multi-layered conductive carbon nanotubes . To understand the structure of nanotubes, we assume that single-walled carbon nanotubes are obtained by rolling graphene sheets . Of course, in practice, the production of nanotubes is done with complex chemical methods. In fact, nanotubes are made by placing individual carbon atoms together.
Conclusion :
The carbon atoms of a graphitic sheet (graphene) form a planar lattice network, in which each atom is connected to three neighboring atoms through strong chemical bonds. Because of these strong bonds, the elastic modulus of graphite base is one of the largest known materials.
Researcher and author: Dr. ( Afshin Rashid)
Specialized doctorate in nano-microelectronics