Section (Self-organized electrical nanostructure)
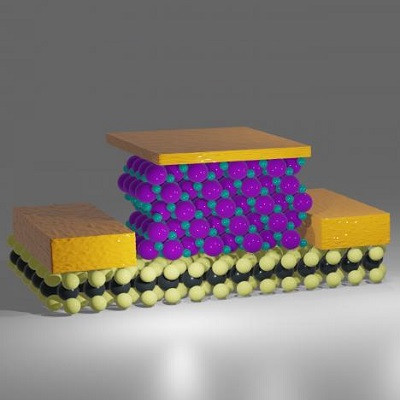
In addition to metallic and covalent bonds , the self-organized electrical nanostructure of DND carbon has an important role in coordination bonds.
Researcher and author: Dr. ( Afshin Rashid)
Note: Nanoparticles in metal and metal oxide clusters and metal/carbon nanocomposites, unlike carbon nanostructures, have an important contribution (in addition to metallic and covalent bonds) in coordination bonds, which causes their system to self-organize. At the same time, the mentioned bonds can increase the change of electronic structure of d metals and thus increase the number of single electrons and the number of magnetic moment of the atom .
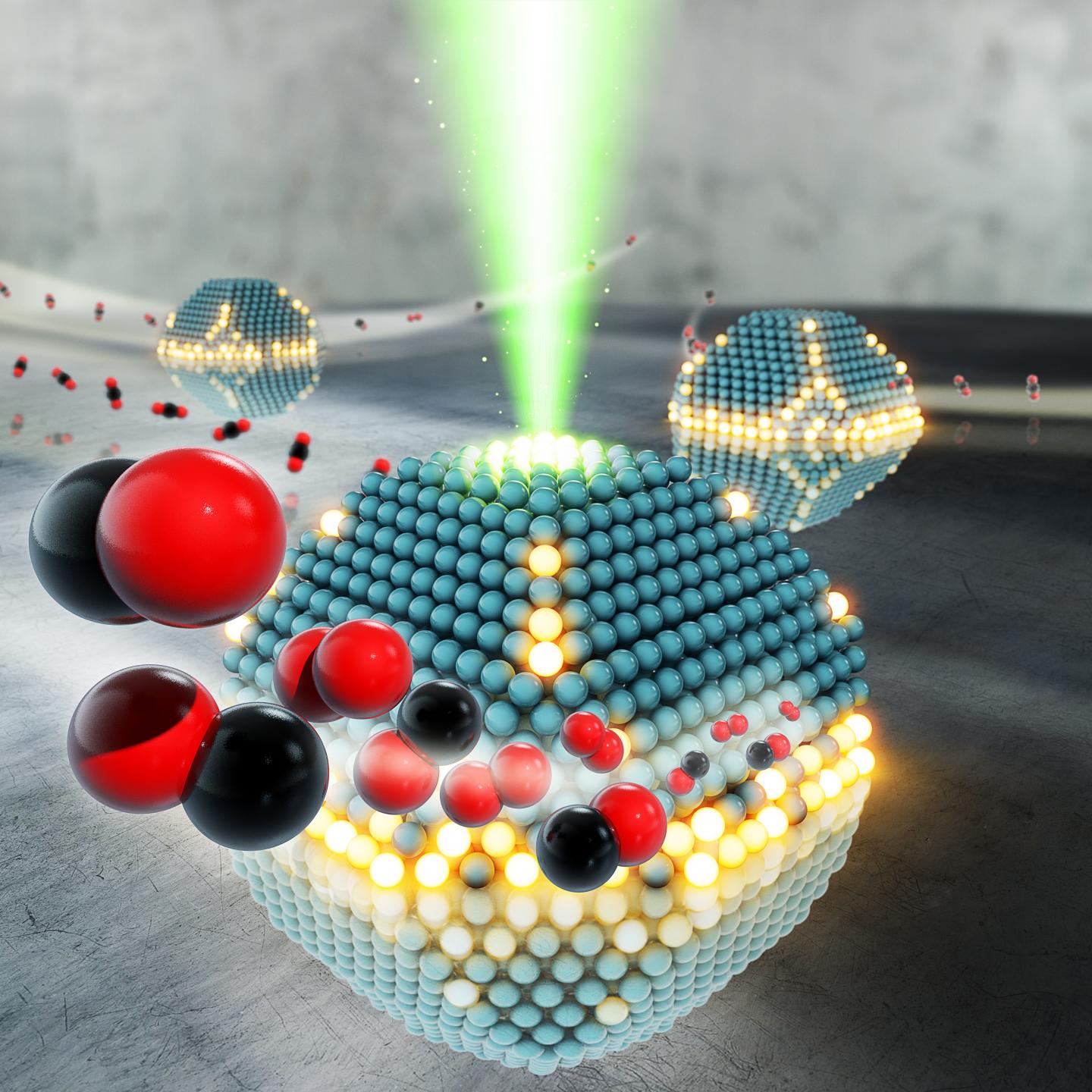
In the manufacture and reproduction of nano devices, the results of nano particle energy growth can be expressed as the total energy of the atoms on the surface of the particle. It is obvious that the freedom of movement of surface atoms is limited and only vibrational movements and the movement of electrons are possible. These two kinetic forms are related to each other because the displacement of electron clouds of atoms definitely changes the vibrational frequencies of bonds of atoms. On the other hand, changing the location of valence electrons in the bonds changes the polarity of the bond and the bodies known as supermolecules, in this case, electron transfer to a higher energy level becomes possible. In this sense, metal/carbon nanostructures are the most interesting species to be investigated. In these nanostructures, metal clusters interact with the carbon envelope that protects them from the environment, and for this reason, these nanostructures are called metal/carbon nanocomposites.
The application of metal/carbon nanoparticles in the form of fine suspensions and sols in certain mediums for the modification of organic and inorganic polymer materials depends on electron paramagnetic resonance, it is possible to design these devices with colors, sizes and properties. The study of the band gap structure of these devices, in addition to introducing a method for the performance of one-dimensional systems, has made it possible to improve the nano-micro electrical properties of electronic components. Devices based on organic materials can be mechanically flexible to a large extent due to the loose intermolecular bonds in the layers created from them. Unlike these organic materials, minerals such as silicon, germanium, and gallium arsenide can be used in the structure of electronic devices only in crystalline states, and in this case, covalent bonds make flexibility impossible.
Conclusion:
Nanoparticles in metal and metal oxide clusters and metal/carbon nanocomposites, unlike carbon nanostructures, have an important contribution (in addition to metallic and covalent bonds) in coordination bonds, which causes their system to self-organize. At the same time, the mentioned bonds can increase the change of the electronic structure of d metals and thus increase the number of individual electrons and the number of magnetic moments of the atom.
Researcher and author: Dr. ( Afshin Rashid)
Specialized doctorate in nano-microelectronics