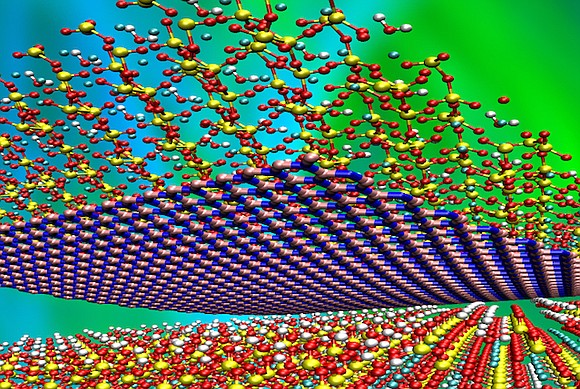
(electrostatic nanoparticles) electrostatic and molecular drift methods for stabilization
Researcher and author: Dr. ( Afshin Rashid)
Note: Due to the specific surface area and high surface energy, the multiplied nanoparticles stick together and form a mass. This phenomenon leads to the loss of properties resulting from the small size of these particles.
To prevent the accumulation of nanoparticles in the synthesis stage, stabilizers are used. Usually, two types of methods, electrostatic and spatial drift, are used to stabilize nanoparticles. In the first method, ions are used to stabilize nanoparticles. These ions are absorbed into the particles and form an electrically charged layer around the nanoparticles, and as a result of the molecular drift of the produced nanoparticles, due to the specific surface area and high surface energy, they stick together and form a mass. This phenomenon leads to the loss of properties resulting from the small size of these particles. To prevent the accumulation of nanoparticles in the synthesis stage, stabilizers are used. Usually, two new methods, electrostatic and spatial drift, are used to stabilize nanoparticles. You see a pattern of two methods of stabilizing particles. In the first method, ions are used to stabilize nanoparticles.
In the second method, macromolecules are used to stabilize nanoparticles. Macromolecules stick to the surface of particles and occupy the space around the particle. As the particles get closer to each other, these molecules get tangled and prevent the particles from sticking together, preventing the accumulation of particles. In the second method, macromolecules are used to stabilize nanoparticles. Macromolecules stick to the surface of particles and occupy the space around the particle. As the particles get closer to each other, these molecules get tangled and prevent the particles from sticking together. In the propagation of nanoparticles by electrostatic regeneration method, they usually use the molecular drift method in order to stabilize the particles. One of the most influencing parameters on the size in the electrostatic synthesis of nanoparticles is the precursor concentration. The higher the precursor concentration, The size of the produced particles is larger, and conversely, the lower the precursor concentration , the smaller the particle size.
Conclusion :
The resulting nanoparticles are used with different stabilizers. Depending on the electrostatic conditions and molecular drift in nanoparticles, each stabilizer can have advantages over other stabilizers in the propagation of nanoparticles. To prevent the accumulation of nanoparticles, the concentration of stabilizers must be within a certain range. A very low concentration of stabilizer cannot prevent the accumulation of nanoparticles, and on the other hand, a high concentration of stabilizer interferes with the production of nanoparticles.
Researcher and author: Dr. ( Afshin Rashid)
Specialized doctorate in nano-microelectronics