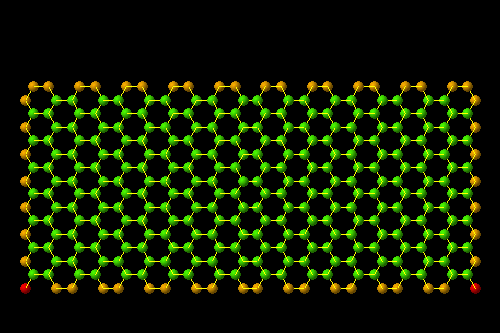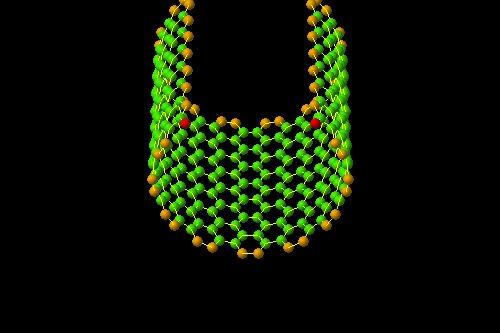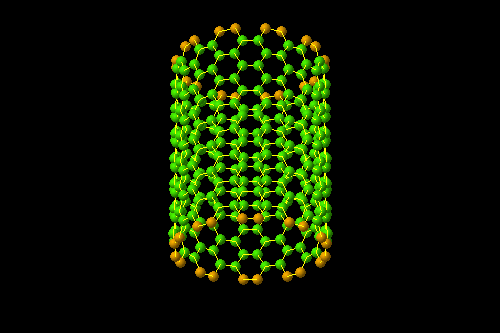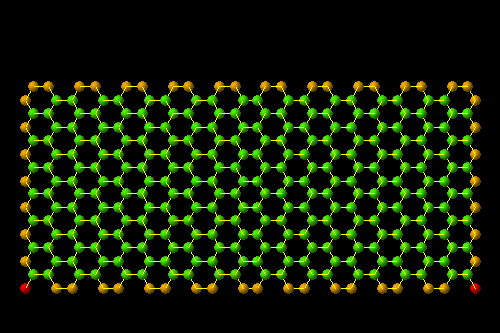
- Section (endohistal nano-fullers)
Investigating electrical properties ( C120 endohistal nano-fullers
Researcher and author: Dr. ( Afshin Rashid)
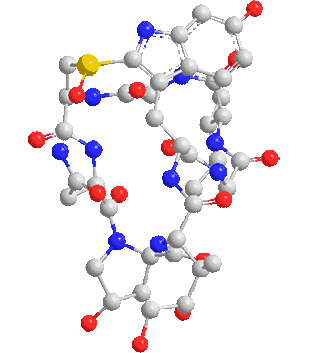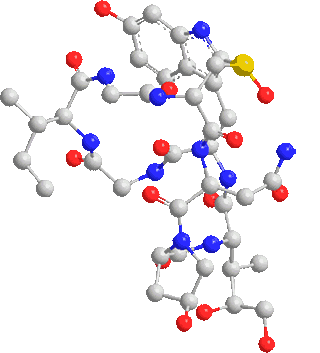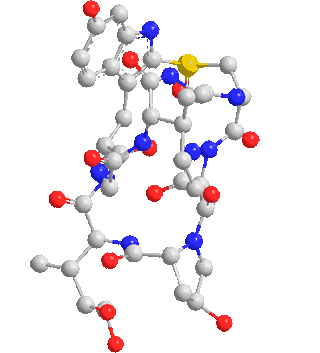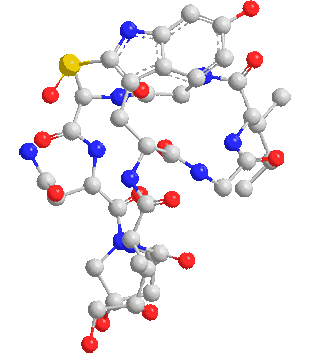
Note: C120 nano fullerenes are among the materials that many nano materials are based on. Their unique structural and electronic properties, as well as their application in various fields such as electronic applications such as the manufacture of nano electrodes used in There are special electric circuits, nano photonics in nano solar cells and nano absorbers of special wavelengths.
One of the most important and unique properties of fullerenes is their ability to keep atoms or small molecules inside the carbon cage. Endohedral carbon cages (EFs), due to their inherent stability, their endohedral nanostructures containing one or two metal atoms such as Sc and La or containing a metal-nitride cluster such as Sc3N have been synthesized. The importance of the spatial and electronic parameters related to the cage structure like fullerene is among the factors of this and the importance of the inclusion of nanostructures and the stability of these compounds. Optical properties, ionization and electron-withdrawing energies, and relative stability of different multiplicities of fleurons are very effective in the reproduction of nanoelectronic devices.
Among the set of fullerenes, C60 and C70 structures are the most abundant. These values include 75 and 24%, respectively, and the remaining 1% is also composed of C74 to C100 fullerenes. The C60 cage with Ih symmetry has a diameter of about 7Å. The C70 molecule is also in the shape of a rugby ball and its symmetry usually has a planar structure, but in fullerenes, the arrangement is 5h, its width is 7Å and its length is 10Å. Bonded carbon The carbon is out of planar form so that the three bonds form a shallow pyramid. The pressure resulting from this change creates an energy difference of about 400 mol/kcal in the C60 molecule, which can be a convincing reason for the greater reactivity of fullerenes compared to nanotubes.
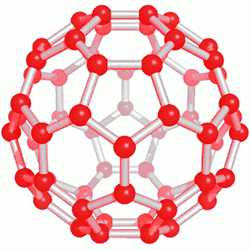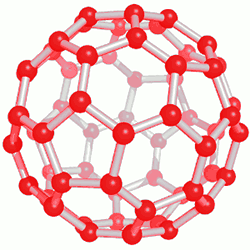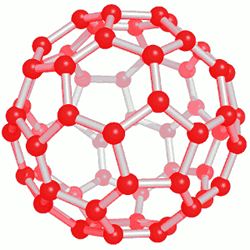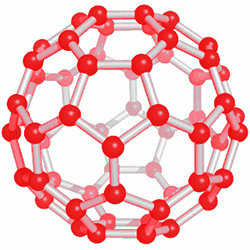
This category of nanostructure compounds was obtained for the first time by electrical discharge method. Of course, the exact mechanism for the formation of fullerenes is not known, but it seems that they are produced during the creation of carbon plasma. Purification of fullerenes is usually done by high-performance column chromatography. Also, on a smaller scale, sublimation can be used to produce solvent-free fullerenes with high purity. Fullerenes are soot-like solids that are dark in color and in some cases bright. The solubility of fullerenes in hydrocarbon solvents is usually small, but it is high in aromatic nanomolecule solvents, and larger fullerenes are less soluble than smaller fullerenes in nanostructured compounds.
Fullerenes are among the materials that many nano materials are based on. Their unique structural and electronic properties, as well as their use in various fields such as electronic applications such as making nano electrodes used in special electrical circuits , nano photonics in nano solar cells and nano absorbers of specific wavelengths.
Researcher and author: Dr. ( Afshin Rashid)
Specialized doctorate in nano-microelectronics