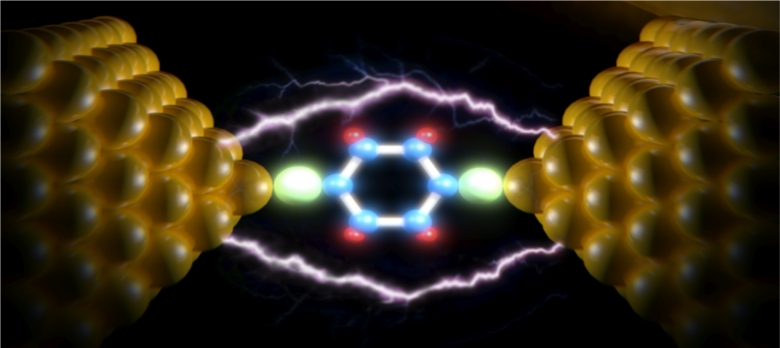
Fullerene molecules in the (nm) Diamond-type fullerenes and fullerene in the second half of electrically conductive nanoparticles in the world (Doctor of nano-micro-electronics)
Researcher and author: Dr. ( Afshin Rashid)
Note: Buckball was the first fullerene to be discovered; The most common properties of fullerenes are C70 and C60 .
Fullerenes are nanometer-sized molecules that, in their simplest form, form 60 carbon atoms of a graphite layer with a three-dimensional structure . 60 Unlike diamonds and graphite, whose molecules are continuous, fullerenes are closed molecules: they are like C60 and so on. (60 fullerenes), also called buccal and buccal tubules, including nanotubes, nanofibers, and fullerenes have a graphite-like structure, but instead of completely hexagonal sections, carbon atoms are also located at the vertices of the 5 (or 7) polygons.
There are two types of bonds in the structure of diamond nano-graphites:
1_ Covalent bond, the bond that exists between the carbon atoms of each honeycomb layer.
2- It is a connection that connects the layers of honey bee to each other.
Nano graphite has high strength in the direction of honeycomb plates due to its strong covalent bond; Conversely, this structure is much less rigid perpendicular to the honeycomb plates. The structure of diamond nano graphite has a lattice structure. In nano-graphite, atomic bonds are established only on the surface, while in the diamond structure, these bonds fill the space as a three-dimensional lattice. In graphite, heratom carbon forms a covalent bond with three other carbon atoms, while in diamond, heratom carbon forms a covalent bond with four other carbon atoms.
In the nanoelectronics industry; Nanochips, Accelerators of Nanotransistors Both types of diamonds, n and p, are also used in nanoelectronics for microelectronics applications. He made blue diamonds and added phosphorus to colorless diamonds to make n-type diamonds. Today, many semiconductors, such as silicon, are used in a wide range of nanoelectronic devices. But nano-diamond, due to its range of thermal changes and extremely high speed, is only compared to gold nanoparticles, which is the second best nanoconductor in the world.
Conclusion:
Nano graphite and graphene nano-strips are conductors of electricity due to the scattering of the π cloud. Active nano diamond particles with such properties, especially electronic ones, can be the founder of a whole new variety of powerful nanoelectronic devices.
Researcher and author: Dr. ( Afshin Rashid)
PhD in Nano-Microelectronics