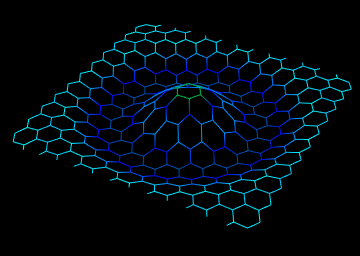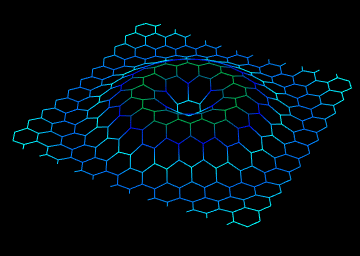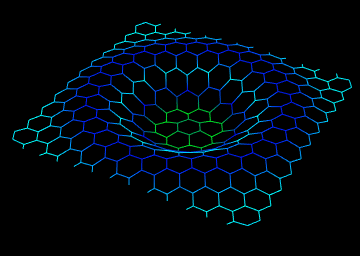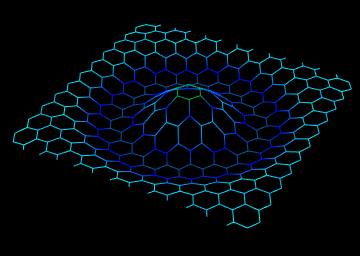
- Oligophenylene vanillin nano wires section
Oligophenylene vanillin nanowires are made by galvanostatic deposition method.
Researcher and author: Dr. ( Afshin Rashid)
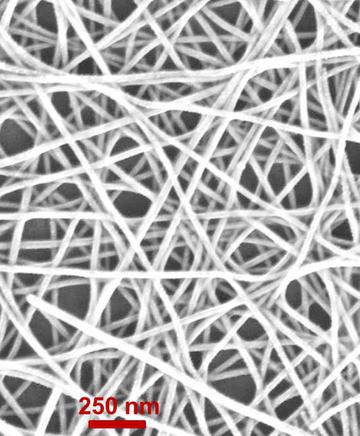
Note: "Oligophenylene vanillin" nanowires with a diameter of about 93 nm are made with the help of electro-accumulation technique on the aluminum mold of nanowires by galvanostatic accumulation method.
Aluminum mold is made through multi-stage (monodizing) pure aluminum sheet. Fabrication of nano arrays of Oligophenylene vanillin wires vertically aligned on flat surfaces and also field emission (FE) using them as electron cathodes . Particles on Au/Ti/Si substrates are obtained at very low temperature (<100°C). After pattern removal, the arrays consist of structurally upright oligophenylene vanillin nanowires with high aspect ratios, uniform dimensions, and predetermined densities. Electron field emission measurements of metallic properties and propagation of cobalt nanowires. In the propagation of cobalt oxide nanotubes, oligophenylene vanillin nanowires embedded in alumina pattern are synthesized in air.
Oligophenylene vanillin nanowires can have insulating, semiconducting or metal properties . Insulators do not carry electric charges, while metals have very good electric charges. Semiconductors lie between the two and are charged under the right conditions. By placing semiconductor wires in a suitable configuration, transistors can be made that either act as switches or amplifiers . Some of the interesting and anti-flexible properties of Oligophenylene vanillin nanowires are due to their small scale. Silicon nanowires are one of the best examples of semiconductor nanostructures that can be made as single crystals with a small diameter of 9 to 0 nm. The electromagnetic nature of nanoparticles in magnetic materials, the molecules and atoms that make them have electromagnetic properties. In other words, elements such as iron, cobalt, nickel and their alloys are attracted by magnets. It is called magnetic material. The classification of electromagnetic materials is based on the magnetic receptivity (magnetization ability of the material). Based on this, materials are classified into three groups: ferromagnetism, paramagnetism, and diamagnetism. Therefore, the dipole moment in electromagnetic diamagnetic materials is zero, and in the presence of a magnetic field, a dipole moment is induced in them; But the direction of these two polarities It is induced against the direction of the external magnetic field, which causes the material (diamagnetism) to be repelled from the magnetic field. By removing the external magnetic field, the magnetic property of these materials does not remain. The magnetic susceptibility of these materials is negative and very low (around 10-6 to 10-3). All gases (except oxygen) are water, silver, gold, copper, diamond, graphite, bismuth and many organic compounds ( diamagnetism). Magnetic dipoles in paramagnetic material do not have a specific and regular orientation; As a result, these materials do not have magnetic properties. If they are placed in a magnetic field, they will be arranged along the magnetic field lines. With the removal of the magnetic field, the magnetic dipoles quickly return to their previous state in the absence of the field. In this way, paramagnetic materials acquire magnetic properties in strong nano-electromagnetic fields.
Conclusion :
Oligophenylene vanillin nanowires with a diameter of about 93 nm are made using the electro-deposition technique on the aluminum mold of nanowires by the method (galvano-static deposition) .
Researcher and author: Dr. ( Afshin Rashid)
Specialized doctorate in nano-microelectronics