Lithium Nanoparticles Nano_Lithim (Structure and Function) PhD Nano _ Microelectronics
Researcher and author: Dr. ( Afshin Rashid)
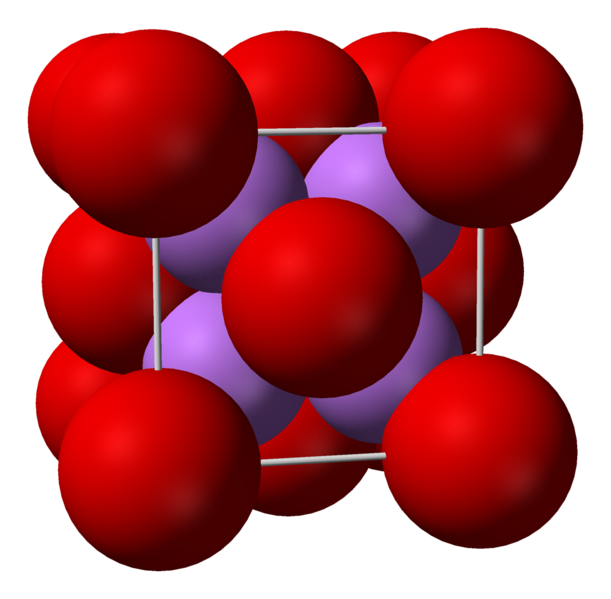
 Note: Lithium with the chemical symbol Li is a silver-white, soft alkali metal with atomic number 3. This element is the lightest metal and the lowest density solid element under standard conditions of temperature and pressure. Like other alkali metals, lithium is highly reactive and flammable, so it is often stored under industrial oil or petroleum. If a cut is made on it, the cut part will have a metallic luster, but due to its high reactivity, it reacts very quickly with the humidity of the air, causing it to corrode and turn dark gray-silver and then black in color. comes.
Note: Lithium with the chemical symbol Li is a silver-white, soft alkali metal with atomic number 3. This element is the lightest metal and the lowest density solid element under standard conditions of temperature and pressure. Like other alkali metals, lithium is highly reactive and flammable, so it is often stored under industrial oil or petroleum. If a cut is made on it, the cut part will have a metallic luster, but due to its high reactivity, it reacts very quickly with the humidity of the air, causing it to corrode and turn dark gray-silver and then black in color. comes.
Due to the high reactivity of lithium, it can never be found as a free element in nature, but is always found in a part of a chemical compound that is mostly ionic. Lithium is found in a number of pegmatite minerals.
Nanoparticles are highly regarded both in industry and in the natural sciences due to their widespread use. While natural materials have fixed physical properties regardless of size, the size of a nanoparticle determines its physical and chemical properties. Therefore, the properties of a substance change as its size approaches the nanoscale and the percentage of atoms on the surface of the material becomes significant. An important feature of all nanostructures is that the number of surface atoms in them is greater than the number of volume atoms. This ratio increases with decreasing nanoparticle size. Therefore, the size of the nanoparticle is an important feature. The range of change in nanoparticle activity depends on the nature and shape of the nanostructure. With However, if the energy field of nano-energy electromagnetic radiation is comparable if certain range Wavelength changes dramatically with the occurrence of chemical reactions in irradiated materials. The activity of nanoparticles up to 100 nm will be significant. Nanoparticle surface atoms are not energy compensated. In general, the growth results of nanoparticle energy can be expressed as the total energy of the surface atoms of the particle. The freedom of movement of atoms on the surface of nanostructures is limited, and only vibrational movements and the movement of electrons are possible. These two electro-kinetic reactions are interdependent because the displacement of the electron clouds of the atoms inevitably changes the vibrational frequencies of the bonds of the nanoparticle atoms . On the other hand, the displacement of capacitance electrons in bonds changes the polarity of bonds and bodies called supermolecules . In this case, the transfer of electrons to a higher energy level is possible.
Conclusion :
Due to the high reactivity of lithium, it can never be found as a free element in nature, but is always found in a part of a chemical compound that is mostly ionic. Lithium is found in a number of pegmatite minerals.
Researcher and author: Dr. ( Afshin Rashid)
PhD in Nano-Microelectronics