Nanoparticles(bimetallic)Description of structure and function (PhD in nano-microelectronics)
Researcher and author: Dr. ( Afshin Rashid)
Note: Metal alloys or bimetallic nanoparticles have high paramagnetic properties that make them suitable for electromagnetic nanomolecules or nanoelectromagnetic carriers . In addition, the electromagnetic properties of the surface of these nanoparticles allow surfactants to be placed on the surface of their nanoparticles that can be used to dissolve the nanoparticles .
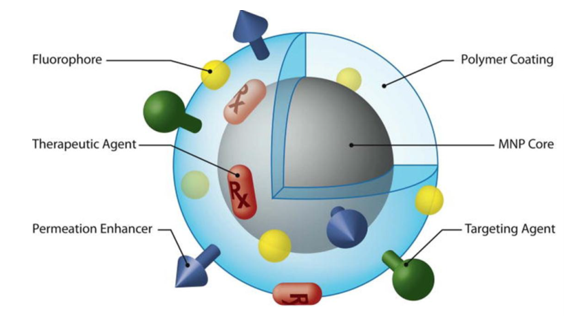
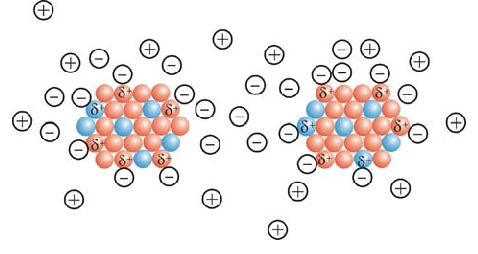
Surface coating is an integral part of electromagnetic nanoparticles that can be used. Although nanoparticles are not attracted to each other due to their paramagnetic supercharger properties , due to the high energy of the surfaces, electromagnetic nanoparticles tend to accumulate and accumulate. Nanomolecular electrostatic stability is not suitable for nanoparticles; Although repelling charges on the surface of nanoparticles can prevent them from accumulating, these charges are neutralized in the presence of a catalyst or other electrolytes inside the electromagnetic nanoparticles.
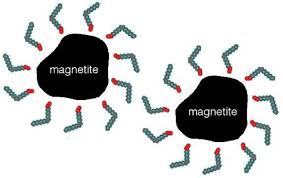
Active electromagnetic properties in nanoparticle coatings prevent them from accumulating as a barrier, and chemical functionalization provides suitable and efficient properties for nanoparticles . Molecular weight and geometric orientation exist on the surface of nanoparticles in various forms. Layers that fully activate electromagnetic nanoparticles. Prevents nanoparticles from accumulating on top of each other. In addition to organic coatings, the core-shell structure is also used for optimal use of electromagnetic nanoparticles . Structural engineering of magnetic nanoparticles is the functionalization of particle surfaces that can have several factors or several (ligands). Uncoated and coated nanoparticles can absorb nanoparticles (bimetallic) with a variety of electromagnetic molecules and create an active process.
Electromagnetic nanoparticles are particles smaller than 100 nanometers that have magnetic properties in the presence of an external magnetic field. The simplest structure of nanoparticles, including a magnetic core (such as iron oxide, nickel, and cobalt), and various non-magnetic coatings of chemical compounds in nano-electromagnetic materials, their constituent molecules, and atoms, are magnetic. Simply put, elements such as iron, cobalt, nickel, and their alloys that are adsorbed by magnets are called nanomagnetic materials.
Conclusion:
The classification of nanomagnetic materials is based on the susceptibility of nanomagnetic particles (the ability of nanoparticles to become magnetic). On the basis of nano-materials into three groups: ferromagnetic, paramagnetic nanoparticles and nano Myknnd.fra diamagnetic classification process dipole moment (metal) and several metal in nano-materials, nano-diamagnetic Active (nano-electro- magnetic) is zero and in the presence of Magnetic field induces bipolar (bipolar) torque ; But the direction of these induced dipoles is opposite to the direction of the external magnetic field.
Researcher and author: Dr. ( Afshin Rashid)
PhD in Nano-Microelectronics