Nano Carbon structure Ph.D. Nano _ Microelectronics
Researcher and author: Dr. ( Afshin Rashid)
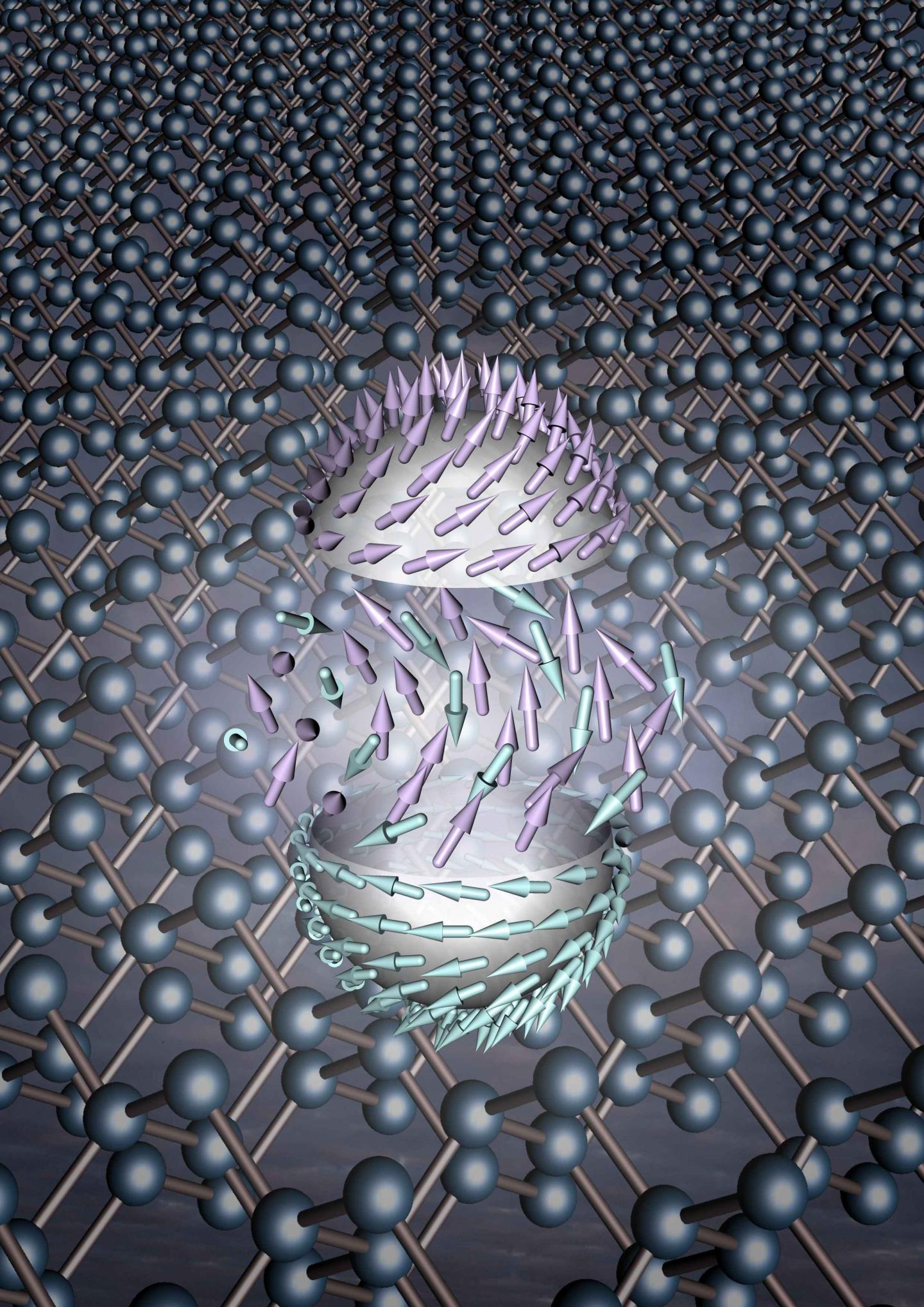
Note: Nanoparticles or nanoparticles in metal and metal oxide clusters and metal / carbon nanocomposites, in contrast to carbon nanostructures, have a share (in addition to metal and covalent bonds (important in coordination bonds) that cause their system to self-organize. At the same time, these bonds can increase the electron structure of metals d, thereby increasing the number of individual electrons and the number of magnetic moments of the atom .
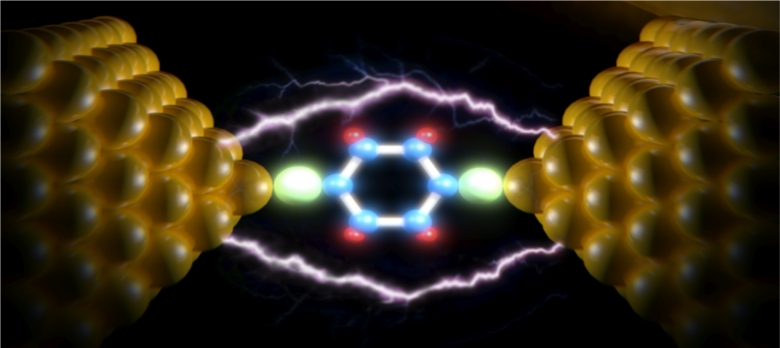
In the manufacture and propagation of nanomaterials, the results of nanoparticle energy growth can be expressed as the energy of all atomic surface atoms. Obviously, the freedom of movement of surface atoms is limited and only vibrational movements and the movement of electrons are possible. These two kinetic forms are interdependent because the displacement of the electron clouds of atoms inevitably changes the vibrational frequencies of the bonds of the atoms. On the other hand, the displacement of the valence electrons in the bonds changes the polarity of the bond and the so-called supermolecule bodies , in which case electron transfer to a higher energy level is possible. In this respect, metal / carbon nanostructures are the most interesting species studied. In these nanostructures , metal clusters interact with the carbon envelope that protects them from the environment, and for this reason these nanostructures are called metal / carbon nanocomposites.
The application of metal / carbon nanoparticles in the form of fine suspensions and sols at certain intermediates to modify organic and inorganic polymeric materials depends on the paramagnetic resonance of the electron, it is possible to combine these devices with colors, sizes and properties. In addition to introducing a method for the performance of one-dimensional systems, the study of the band gap structure of these devices has made it possible to improve the nano-microelectric properties of electronic components. Organic-based devices can be largely mechanically flexible due to the weak intermolecular bonds in their layers. Unlike these organic materials, minerals such as silicon, germanium, and gallium arsenide can only be used in the structure of electronic devices in crystalline states, in which case covalent bonds make flexibility impossible.
Conclusion :
Or nanoparticles Nanoparticle in clusters of metal and metal oxide nano-composites and metal / carbon-carbon nanostructures reverse share) in addition to the metallic bonds and covalent (Kyvrdynansy important links) that leads to self-organization of the system. At the same time, these bonds can increase the change in the electronic structure of d metals, thereby increasing the number of single electrons and the number of atomic magnetic moments.
Researcher and author: Dr. ( Afshin Rashid)
PhD in Nano-Microelectronics