(nuclear power)
Nuclear power - different types of uranium in the uranium industry and nuclear power in the electric energy production industry
Researcher and author: Dr. ( Afshin Rashid)
Note: There are three types of uranium in the uranium industry and nuclear power in the electrical energy production industry:
• Alpha (Orthohombic) which is stable up to 667.7 degrees.
• Beta (Tetragonal) which is stable from 667.7 to 774.8 degrees.
• Gamma (Body-centered cubic), which is stable from a temperature of 774.8 degrees to the melting point. (This is the most conductive and malleable form of uranium.)
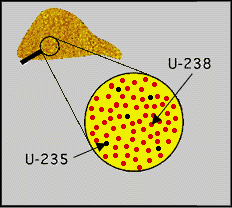
Its two important isotopes are U235 and U238>, which U235 is the most important for nuclear reactors and weapons. Because this isotope is the only isotope that exists in nature and is split by thermal neutrons in every possible amount. The U238 isotope is also important because it absorbs neutrons to produce a radioactive isotope and decomposes it into the Pu239 plutonium isotope. The synthetic isotope U233 is also fissioned and created by neutron bombardment of Thorium232. Uranium was the first element discovered that could be fissile. For example, with the slow neutron bombardment of the isotope U235, it turns into the short-lived isotope U236 and immediately splits into two smaller nuclei, which releases energy and produces more neutrons. If these neutrons are absorbed by another U235 nucleus, the nuclear ring action occurs again and if there is nothing to absorb the neutrons.
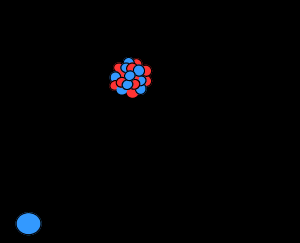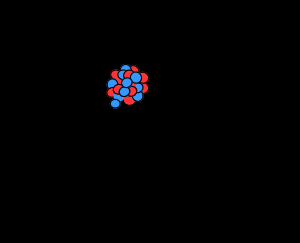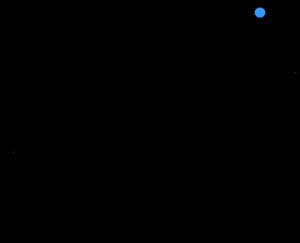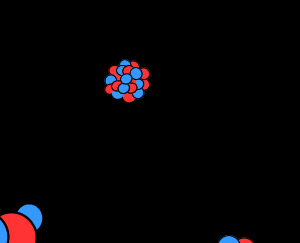
Protons have a positive electric charge and neutrons have no electric charge (they are neutral), while electrons have a negative electric charge. An electron is a subatomic particle with a negative charge. It can be free (not attached to any atom), or attached to the nucleus of the atom. Electrons in atoms exist in spherical shells with different radii that represent energy levels. The larger the spherical shell, the more energy in the electron. In electrical conductors, the current results from the movement of electrons from atom to atom individually and generally from negative to positive electrical poles. In semiconductor materials, current also occurs as a movement of electrons. But in some cases, it makes more sense to imagine the flow as a movement of electron deficiency from atom to atom. An electron deficiency atom in a semiconductor is called a hole . Holes generally move from positive to negative electrical poles. The charge of an electron is considered as unit electric charge. It is assigned the negative pole. The charge on an electron is equal to the positive charge on a proton or hole, but opposite. The quantity of electric charge is usually not measured in terms of the charge of an electron.To produce electricity, nuclear fission in a reactor is only part of a nuclear cycle. This cycle starts from mines. Uranium extracted from the mine usually has a stable and compact form like yellow cake. This mineral uranium is sent to processing facilities, where the yellow cake is converted into uranium hexafluoride (which is used as reactor fuel after enrichment). At this stage, the degree of uranium enrichment, i.e., the percentage of uranium-235, is around 0.7%.
Conclusion:
Electrons in atoms exist in spherical shells with different radii that represent energy levels. The larger the spherical shell, the more energy in the electron. In electrical conductors, the current results from the movement of electrons from atom to atom individually and generally from negative to positive electrical poles.
Researcher and author: Dr. ( Afshin Rashid)
Specialized doctorate in nano-microelectronics