Nanoparticles and nanostructures (Fullerenes), (CNT nano-tubes) based on nano-microelectronics (PhD)
Researcher and author: Engineer Afshin Rashid
Note: Carbon nanotubes (CNTs) exhibit special physicochemical properties such as thermal, magnetic, optical or electrical conductivity.
Biological reactions and toxicity of carbon nanotubes, and CNT nanotubes, as with other nanomaterials, have been shown to have several physical properties such as length, diameter, surface area, tendency to condense, dispersion, presence and nature of residual catalysts. As well as the chemical functionality of these nanomaterials depends on these causes, and especially considering the growing trend of carbon nanotubes in biosensors and the study of CNT toxicity in biological environments and the attempt to address this problem in general, in biosensors. environment and biological interaction with the CNT electronic properties and interfere with their structure Occurs, it is likely that the toxicity of carbon nanotubes the electrons in nano-porous structures is reduced . One way to reduce the toxicity of CNT It is suggested to use surface coatings on nanotubes . Putting Functional Functional Groups in the Functionalization Technique It has been shown in various studies that exposure to pure carbon nanotubes significantly reduces cell proliferation and cell cycle, apoptosis and necrosis in the structure of biosensors and Biosensors can be separated and coated with specific molecules as a result of the functionalization process of carbon nanotubes and biosensors that typically accumulate in a cluster . Carbon nanotubes (2600 g / 2) are poly aromatic compounds with a surface area greater than m . The side surfaces of the nanotubes are very hydrophobic.
Nanoparticles and nanostructures (fullerenes)
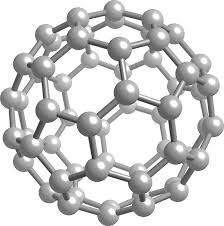
Structure and structure In nature, there are five allotropes of diamond, graphite, nanotubes, amorphous carbon and fullerenes, all of which are solid. The basis of the fullerenes is the graphite plates, but in the atomic structure of the fullerenes instead of the regular hexagons in the graphite plates, there is a series of hexagons and regular hexagons that are interconnected. And made up of fullerenes. Putting these hexagons and hexagons together is essential to form a spherical structure. In fact, without the presence of pentagons in the graphene structure, the graphene plates of the spherical structures cannot be obtained.
The fullerenes are identified by the number of atoms in their building. A letter C is used to name the fullerenes, which denotes the carbon atom present in the structure. After the letter C, the number of carbon atoms in the fullerenes spherical lattice unit is given. For example, the C60 molecule has 60 carbon atoms. The number of atoms in the fullerene produced so far ranges from 28 to hundreds of carbon atoms