Electro-static and Molecular Drift Method for Stabilizing Nanoparticles (Based on Nano-Microelectronics PhD)
Researcher and author: PhD student Afshin Rashid
Note: Duplicated nanoparticles stick together to form a mass due to their high surface area and high surface energy. This phenomenon results in the loss of properties due to the small size of these particles.
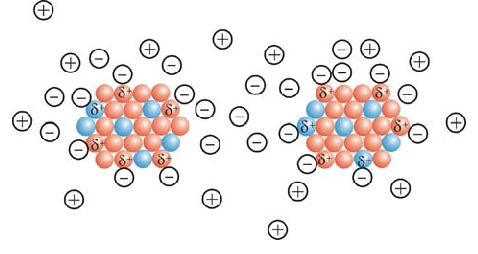
Stabilizers are used to prevent the accumulation of nanoparticles at the Sunz stage. Typically, two types of electrostatic and spatial drift methods are used to stabilize the nanoparticles. In the first method, ions are used to stabilize the nanoparticles. These ions are attracted to the particles and form an electrically charged layer around the nanoparticles, resulting in the molecular drift of the nanoparticles produced due to their special surface area and high surface energy, forming a mass. This phenomenon destroys the properties of the small size of these particles. Stabilizers are used to prevent the accumulation of nanoparticles during the synthesis phase. Two methods of electrostatic and spatial drift are commonly used to stabilize nanoparticles. You can see a pattern of two methods of stabilizing the particles. In the first method, ions are used to stabilize the nanoparticles.
In the second method, the molecules are used to stabilize the nanoparticles. The large molecules attach to the surface of the particle and occupy space around the particle. As the particles approach each other, these molecules tangle and prevent the particles from sticking together. In the second method, the molecules are used to stabilize the nanoparticles. The large molecules attach to the surface of the particle and occupy space around the particle. As the particles approach each other, these molecules intertwine and prevent the particles from adhering. In nanoparticles propagated by electrostatic resuscitation, molecular drift is usually employed to stabilize particles. One of the most important parameters affecting size in the electrostatic synthesis of nanoparticles is the precursor concentration. The higher the precursor concentration, The larger the particle size, and the lower the precursor concentration , the smaller the particle size.
Conclusion:
The resulting nanoparticles are used in various stabilizers. Depending on the electrostatic conditions and the molecular drift of the nanoparticles, any stabilizer can have advantages over other stabilizers in the nanoparticle proliferation. To prevent the accumulation of nanoparticles, the concentration of stabilizers must be within a certain range. Very low stabilizer concentration cannot prevent the accumulation of nanoparticles, and on the other hand, high stabilizer concentration can impair the production of nanoparticles.
Author: Engineer Afshin Rashid
PhD student of Nano-Microelectronics at Islamic Azad University, Science and Research Branch, Tehran