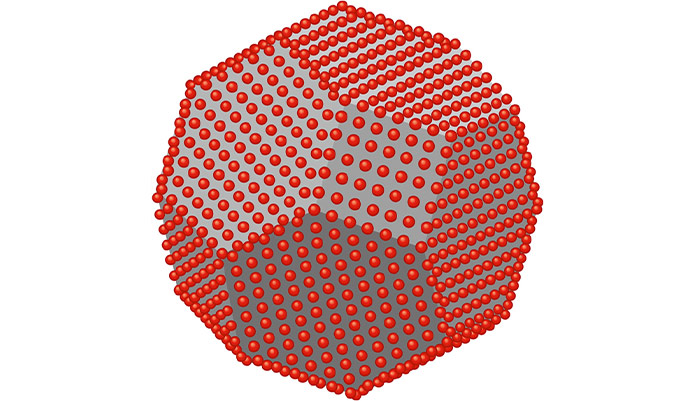
Diamond nano particles Structure and performance (PhD in nano-microelectronics)
Researcher and author: Dr. ( Afshin Rashid)
Note: Diamonds are made from only one element found in nature, carbon. Diamonds have a very compact atomic lattice that causes high hardness, or in other words, the hardest crystallized material on Earth. The chemical formula of diamond is pure carbon (C) , but detailed studies show that 99.95% of it is carbon and the rest is chemical impurities, the same small amount of which can affect the color of diamond.
Nano carbon has many applications in nanoscience and nanoelectronics. Carbon is one of nature's most amazing elements, found in nature in four different forms: graphite, diamond, coal, and other forms of carbon. All four of these shapes are solid, and in their structure the carbon atoms are arranged next to each other in a complete and orderly manner. Carbon is one of the most important elements in nature and its many applications in human life, well confirms this point. Steel, for example, which is one of the main engineering alloys, is obtained by dissolving about two percent of carbon in iron; By changing the percentage of carbon to only a few hundred percent, all types of steel can be obtained. "Organic chemistry" is also a science that studies compounds containing "carbon" and "Hydrogen" and polymer engineering is based solely on the element carbon. Carbon is found in four different forms in nature , all of which are solid forms, and in their structure carbon atoms are arranged next to each other in a completely regular manner.
Diamond nanoparticles are one of the most advanced carbon materials in the world. Diamond is known for its extraordinary physical properties such as the highest hardness on earth, superior thermal conductivity over any bulk material, high refractive index and number of voids. Diamond nanoparticles are not only small diamonds with the above characteristics, but due to the size of the nanoparticle, different functional groups, high dispersion, z-potential and amorphous carbon can create other functions as well. The structure of diamond nanoparticles consists of a diamond core and amorphous carbon layers. In more complex terms, each particle of the nanodiamond has an SP 3 bond of carbon atoms covered by SP 2 . The most unique feature of nano-dimensional is the presence of different oxygenated functional groups on the surface, so diamond nanoparticles They have high potential in water. High potential disperses diamond nanoparticles evenly and stably in water. Diesel also dispersed nanofields in various organic solvents such as IPA, THF, MIBK and toluene by modifying surface chemicals on nanomasses. The nano-field is produced by the blasting method and the synthesized diamond is a very fine particle with an average diameter between 4.6 nm. Unlike abrasive diamonds, these particles are almost spherical (exactly polygonal) for lip, creep, and polish. Depending on the size and shape, a certain surface area of a nanodiamond particle over 300m is 2 / g. Diamond nanoparticles can be used in unique applications compared to other nanomaterials such as nanosilica, graphene, carbon nanotubes, fullerenes and carbon nanotubes.Possible applications of nanomaterials are additives for degreasing lubricants, antioxidants, grain purifiers, bioimaging, drug delivery, diamond sensors and more. Each performance of diamond nanoparticles in applications is remarkably unique. Diamond nanoparticles can be an excellent modifier nanomaterial with many potential products and applications.
Conclusion :
Diamonds have a very dense atomic lattice that causes high hardness, or in other words, the hardest crystallized material on Earth. The chemical formula of diamond is pure carbon (C) , but detailed studies show that 99.95% of it is carbon and the rest is chemical impurities, the same small amount of which can affect the color of diamond.
Researcher and author: Dr. ( Afshin Rashid)
PhD in Nano-Microelectronics