رشید)
Note: All the atoms in Nano Structures at any temperature allocate a certain amount of energy due to their fluctuations. The amplitude of this oscillation is not the same in all the atoms of the nanostructures.
Moreover, the surface atoms have a larger range of oscillation due to the greater spatial freedom they have. In this way, the strange behavior of solids in reducing their melting temperature can be explained. When the average value of the atomic vibration range reaches a certain coefficient of the constant value of the network of nanostructures, these vibrations can no longer increase without damaging and destroying the nanoparticle network. Therefore, by increasing the average amplitude of vibrations to larger values, the nanostructures are removed from the crystal lattice and melt. Surface atoms have higher average fluctuations and if the number of surface atoms increases, they can have a clear effect on the average range of fluctuations of all atoms of the material. Therefore, by shrinking the dimensions of nanostructures until the ratio of the number of atoms on the surface to the number of atoms in the volume reaches a significant value, the average range of nanomolecular oscillations will increase significantly; In this situation, with the increase of the surface instability of the material, the melting temperature of Nano Nano Structures will decrease.
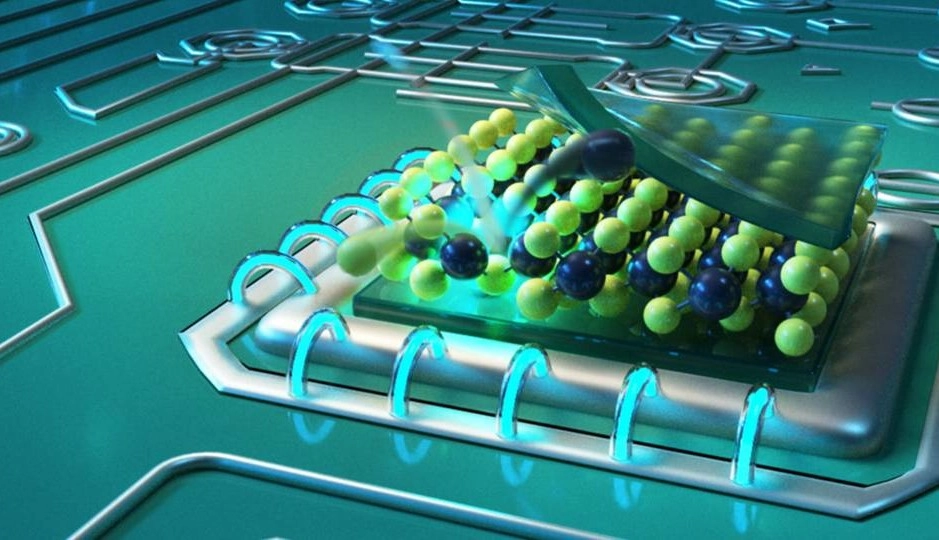
In the change (structure) of nano structures, Nano Structure is one of the natural thermal phenomena caused by the shrinking of the material structure, the thermal instability of nanoparticles. As you know, heat has energy E, which 03- the amount of energy is proportional to the temperature of the environment. This energy is equal to KBT. In this relation, KB is Boltermann's constant and a constant value equal to and T is the temperature in Kelvin. Therefore, when the nanostructures are placed in different environments, due to heat, the electrons are given energy (JK-0.01*0.33). Now consider a property of a particle that generally depends on (the volume of the nanoparticle V). The energy of this feature is U and a function of V. When the volume is small enough, so that KBT is greater than U, then the conditions will be thermally unstable. The important feature of all nanostructures is summarized in the fact that the number of (surface) atoms in them is proportional to The number of atoms (volume) is greater. This ratio increases with decreasing size (nanoparticle). Therefore, the size of the nanoparticle is considered its most important feature. The shapes and sizes of nanostructures are naturally determined based on their composition and formation conditions. The characteristics of the nanostructures, in turn, determine the originality of the characteristics of the nanostructures and their possible fields of operation. The range of 1 to 1000nm is introduced as the range of nanostructures, the important characteristic of nanostructures is the control of their processes. organization. The range of nanostructure activity change depends on the nature and shape of the nanostructure. However, if the energy of the nanoparticle field is comparable to the energy of electromagnetic radiation and if significant changes are made in a certain wavelength range with the occurrence of chemical reactions in the irradiated materials, the activity of nanoparticles up to 100nm will be significant.
Conclusion :
All the atoms in Nano Structures at any temperature allocate a certain amount of energy due to their fluctuations. The amplitude of this oscillation is not the same in all the atoms of the nanostructures.