Investigation of Granular Nano Graphene Growth Structure on SiC Surface
Researcher and author: Dr. ( Afshin Rashid)
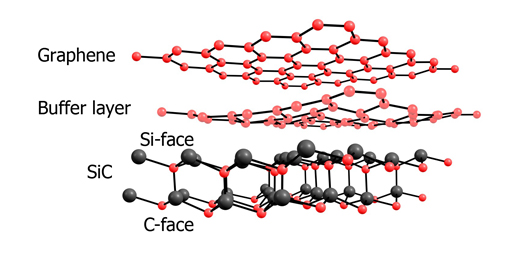
Note: In the epitaxial growth method on SiC surface at high temperature, single-layer or multi-layer graphene can be obtained. This method requires a high temperature of more than 1100 degrees Celsius. At this temperature, the carbon source decomposes.
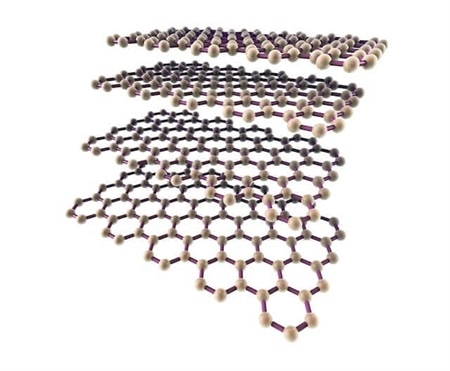
Graphene is a two-dimensional form of carbon atoms with sp hybrid , which are located in a hexagonal lattice structure and is known as the thinnest material with a thickness of 1.95 nm. Graphene plates are formed by placing carbon atoms together. On a graphene plate, each carbon atom is bonded to nine other carbon atoms. These three links are on the same plane and the angles between them are equal and equal to 421 °. In this case, the carbon atoms are positioned to form a network of regular hexagons in an ideal state. The length of the carbon-carbon bond in graphene is about 1.412 nm. In a graphene plate, each carbon atom has an orbital outside the plate. This orbital is a good place to connect with some groups Factor as well as hydrogen atoms. The bond between the carbon atoms in the covalent plane is very strong. Therefore, graphene is very strong and carbon nanotubes are expected to be very strong as well. Graphite is also a well-known and widely used carbon material that is formed by overlapping layers of graphene to form a regular structure. Another way to produce graphene is to separate the graphite layers to make graphene. What holds the graphene layers together is the van der Waals bonds between them. This link is very weak. Therefore, graphene layers can easily slide on top of each other. Graphene layers from 9 to 41 layers are called low-layer graphene and between 41 and 91 layers are called multi-layer graphene , 6 thick graphene or thin graphite nanocrystals.
Because the rate of sublimation of silicon is higher than that of carbon, excess carbon remains on the surface, forming carbon nanofilms after rearrangement. These substances are called coke. These materials are considered a catalytic toxin, so the synthesis of graphene in this way is not suitable for catalytic applications . Another weakness of this method is the large structural defects in the layers. In addition it is difficult to transfer to other substrates . It also requires high vacuum conditions and is a costly method.
Conclusion :
The size and physical shape of nanomaterials and how they are interatomically bonded are important parameters that greatly affect the properties of materials in nanometer dimensions. Length, diameter, arrangement of atoms in the structure of nanotubes, structural defects, number of walls are among the important factors that affect the properties of nanotubes. Carbon nanotubes Carbon plates are one atom thick and hollow in a cylindrical shape , and the arrangement of carbon atoms in the wall of this cylindrical structure is exactly the same as the carbon structure in graphite plates. In graphite, regular carbon hexagons are stacked next to each other. If we cut a single-layer nanotube lengthwise, we get a plate of carbon atoms called graphene. The latest metamorphosis to be discovered is graphene carbon.
Researcher and author: Dr. ( Afshin Rashid)
PhD in Nano-Microelectronics