Crown nanomolecules for graphene synthesis of carbon nanotubes (nano _ PhD in microelectronics)
Researcher and author: Dr. ( Afshin Rashid)
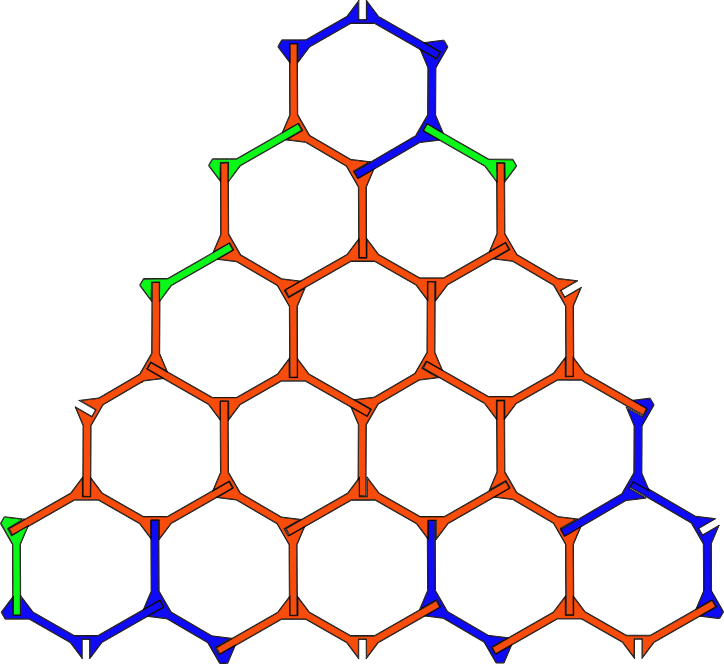
Note: In this method, graphene nanofibers are created directly inside single-walled carbon nanotubes.
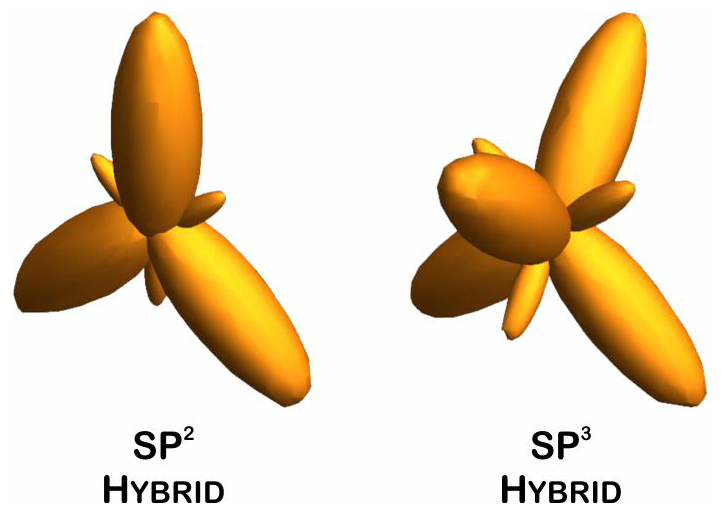
Graphene is a material that has a high efficiency due to its unusual properties. This material has a very high electrical and thermal conductivity. By transforming graphene into thin strips called nano-ribbons, this material acquires various properties. Nanofibers have great application potential in the nanoelectronics industry . However, producing these structures is not an easy task. Graphene is a two-dimensional form of carbon atoms with hybrid sp2 and three-dimensional SP3, which are located in a hexagonal lattice structure and with a thickness of 1.95 nm is known as the thinnest material. Graphene plates are formed by the carbon atoms together . On a graphene plate, each carbon atom is bonded to nine other carbon atoms. These three links are on the same page The angles between them are equal and equal to 421 °. In this case, the carbon atoms are positioned to form a network of regular hexagons in an ideal state. The length of the carbon-carbon bond in graphene is about 1.412 nm. In a graphene plate, each carbon atom has an orbital outside the plate. This orbital is a good place to bond with some functional groups as well as hydrogen atoms. The bond between the carbon atoms in the covalent plane is very strong.
Graphite is also a well-known and widely used carbon material that is formed by overlapping layers of graphene to form a regular structure. Another way to produce graphene is to remove enough layers of graphite to turn it into graphene. What holds the graphene layers together is the van der Waals bonds between them. This link is very weak. Therefore, graphene layers can easily slide on top of each other. In this method, first graphene oxide is created and then it is converted to graphene by reducing agents. In this method, mainly graphene oxide is not completely reduced, so instead of graphene, reduced graphene oxide is obtained.
Oxidation of graphite layers in this way significantly reduces the interaction between them and increases the distance between the layers. Graphene oxide and reduced graphene oxide obtained by this method have many structural and chemical defects, so this method is a suitable method for the synthesis of graphene oxide and reduced graphene oxide. Graphene has unique properties due to its special structure. Graphene is one of the strongest materials known in terms of strength . Graphene is 211 times stronger than steel. Some of the special properties of graphene that make it a popular material .
Conclusion :
Croene molecules can be used to synthesize graphene but need to be aligned on a plate to provide reaction conditions. The interior of carbon nanotubes seems to be suitable for this purpose. Metal nanotubes inside insulated nanotubes are insulated wires. These wires can be inserted directly into the nanotubes.
Researcher and author: Dr. ( Afshin Rashid)
PhD in Nano-Microelectronics