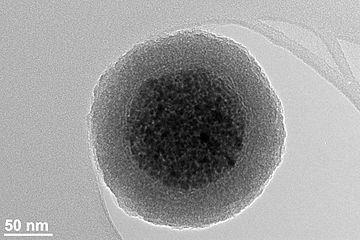
Chromium (Cr) Nanoparticles Structure and Function (PhD in Nano-Microelectronics)
Researcher and author: Dr. ( Afshin Rashid)
Note: Chrome or chromium is a hard, shiny metallic gray with a high gloss and high boiling point and significant resistance to corrosion and dullness. Chromium is one of the elements of the periodic table that has the symbol Cr and atomic number 24. The use of particles from micro-scale to nanoscale offers advantages for various scientific fields, but because a large percentage of their atoms are on the surface, nanomaterials can react greatly and pose potential risks to humans. To have with.
Cr ( Chromium) in the form of chromite ore (H2CrO4) is extracted. The most common oxidation states of chromium are +2, +3, and +6, with +3 being the most stable and +4 and +5 being relatively "rare." Chromium compounds in oxidation state 6 are strong oxidizers. Chromium is naturally composed of 3 stable isotopes and 19 radioisotopes. This element also has 2 excited states. Nanoparticles are highly regarded both in industry and in the natural sciences due to their widespread use. While natural materials have fixed physical properties regardless of size, the size of a nanoparticle determines its physical and chemical properties. Therefore, the properties of a substance change as its size approaches the nanoscale and the percentage of atoms on the surface of the material becomes significant.An important feature of all nanostructures is that the number of surface atoms in them is greater than the number of volume atoms. This ratio increases with decreasing nanoparticle size. Therefore, the size of the nanoparticle is an important feature. The range of change in nanoparticle activity depends on the nature and shape of the nanostructure. However, if the nanoparticle field energy is comparable to the electromagnetic radiation energy, and if within a certain wavelength range chemical reactions occur in the irradiated material, the activity of nanoparticles up to 100 nm will be significant. Nanoparticle surface atoms are not energy compensated.
In general, the growth results of nanoparticle energy can be expressed as the total energy of the surface atoms of the particle. The freedom of movement of atoms on the surface of nanostructures is limited, and only vibrational movements and the movement of electrons are possible. These two electro-kinetic reactions are interdependent because the displacement of the electron clouds of the atoms inevitably changes the vibrational frequencies of the bonds of the nanoparticle atoms . On the other hand, the displacement of capacitance electrons in bonds changes the polarity of bonds and bodies called supermolecules . In this case, the transfer of electrons to a higher energy level is possible.
Conclusion :
Chrome or chromium is a hard, shiny metallic gray metal with high gloss and high boiling point and significant resistance to corrosion and dullness. Chromium is one of the elements of the periodic table that has the symbol Cr and atomic number 24. The use of particles from micro-scale to nanoscale offers advantages for various scientific fields, but because a large percentage of their atoms are on the surface, nanomaterials can react greatly and pose potential risks to humans. To have with.
Researcher and author: Dr. ( Afshin Rashid)
PhD in Nano-Microelectronics