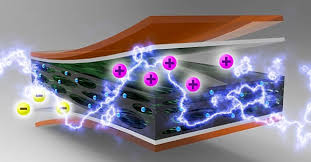
(electrical nanoparticles) electrostatic interaction of graphene nanoparticles
Researcher and author: Dr. ( Afshin Rashid)
Note: Graphene nanoparticles, due to their high electrical properties, as well as the abundance of their main component i.e. graphite in nature, are considered a suitable alternative to carbon nanotubes in order to produce electronic nanostructures.
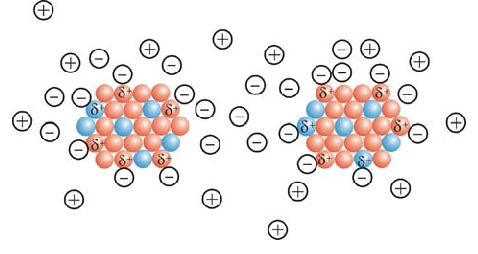
On the other hand, due to the unique properties of graphene, including nano-electrical , nano-electrochemical properties and high specific surface area, the ability to use this material in many applications such as sensors, nano-transistors, energy storage sources and all kinds of nano-devices Microelectronics has grown dramatically. Graphene has significant nanoelectronic properties, including; high Young's modulus; High fracture strength , excellent nanoelectrical conductivity, fast movement of (nanobars) . There is a simple electrostatic interaction between graphene nanoparticles in a solution, graphene nanoparticles like semiconductors, nanotransistors.
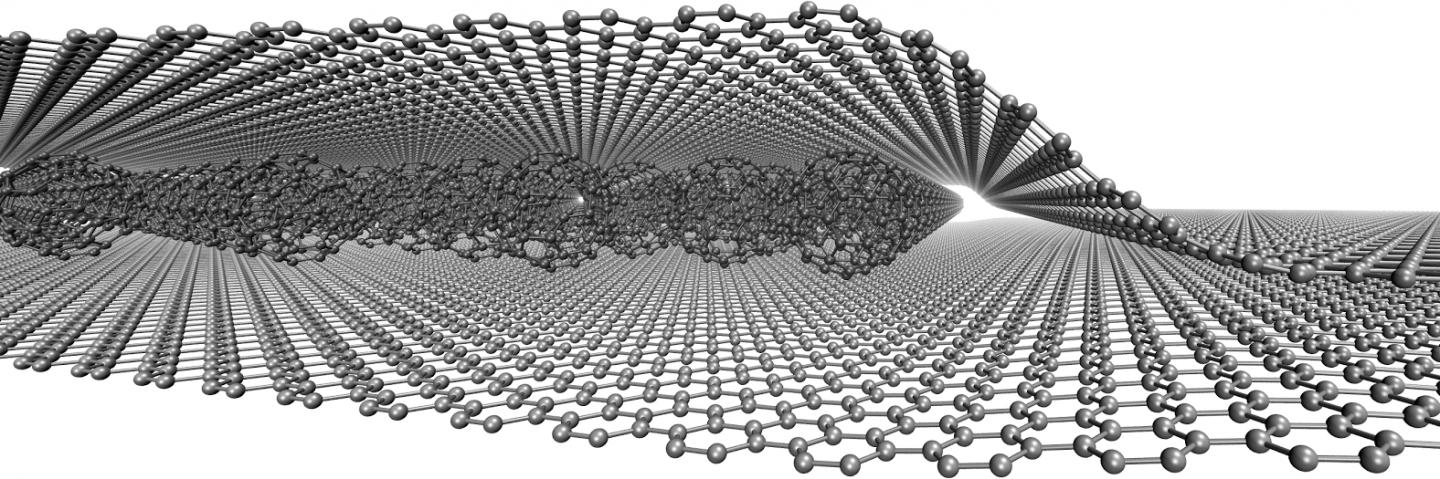
In terms of building blocks, graphene is fundamentally different from other two-dimensional or three-dimensional graphite materials and other geometric forms of carbon, such as zero-dimensional spherical fullerene. Graphene is made of one layer with one-dimensional carbon nanotubes from a hexagonal ring, which can be proposed as a supermolecule of aromatic planar conjugation. The planar structure of graphene has created an excellent ability to use this material in the field of transfer and electrostatic interaction in nano-electrical devices.
The application of graphene nanoribbons in nano electromagnetic shielding materials and nano electrostatic discharge in nano electronic devices has been expanded to Rectenna nano antennas, and nano supercapacitors. The effectiveness of graphene particles in electromagnetic interference shielding and enhanced with modified graphene nanoribbons is measured. One of the important and new structures of carbon nanostructures is graphene. Graphene is a sheet of carbon atoms. Graphene is the newest member of the large carbon family. The term graphene was used to describe a single nano layer of graphite. This nano material has high transparency due to its small thickness. Also, its conductivity has caused it to attract a lot of attention. It is very difficult to control the conditions for separating a graphene monolayer, usually material The preparation, called graphene, consists of collections of several layers of graphene , each containing a different number of sheets.
Conclusion :
The electrostatic interaction of graphene nanoparticles takes place on a non-planar molecular surface, and there is no interacting nanographene with a completely flat atomic structure, graphene sheets are flexible, that is, they bend, fold, or their surface becomes wavy. The large bends are related to the method of preparing graphene, and the small waves are the inherent properties of separated layers .