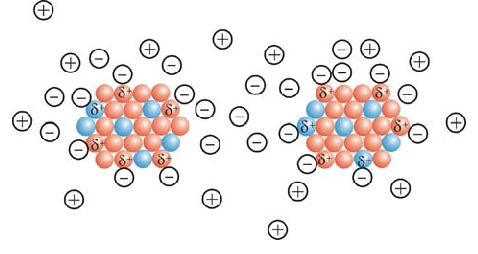
(electrical nanoparticles) coating agent or stabilizer in nanoelectronics
Researcher and author: Dr. ( Afshin Rashid)
Note: Due to the specific surface area and high surface energy, the coating or stabilizing agent of the produced nanoparticles stick together and form a mass. This phenomenon leads to the loss of properties resulting from the small size of these particles.
To prevent the accumulation of nanoparticles in the synthesis stage, stabilizers are used. Usually, two types of electrostatic and spatial drift methods are used to stabilize nanoparticles. In this model, two methods of stabilizing particles are used. In the first method, ions are used to stabilize nanoparticles. These ions are absorbed into the particles and form an electrically charged layer around the nanoparticles, and as a result, the Raman covalent drift becomes from the accumulation of particles. In the second method, macromolecules are used to stabilize nanoparticles. Macromolecules stick to the surface of the particles and occupy the space around the nanoparticle. As the particles get closer to each other, these molecules become entangled and become Raman from the particles sticking together. It is from the interactions between nano layers and fat as a function of nanoparticle structure, morphology and surface electrochemistry. This broad target consists of a number of nanomolecules obtained from coarse-grained molecular simulations.
In the production of porous nanoparticles by the method of regenerating the coating agent or nanoparticle stabilizer, they usually use the space drift method in order to stabilize the particles. In this method, a mixture of two stabilizers is used as a stabilizing agent. They are most widely used in the coating of nanoparticles. In the synthesis of nanoparticles based on the reducing agent, stabilizer and along with the size of the nanoparticles, it has been obtained. One of the most influencing parameters on the size in the synthesis of nanoparticles is whether the concentration of the monitoring substance or precursor concentration is higher, the size of the produced particles will be larger, and conversely, the lower the concentration of the monitoring substance , the smaller the size of the particles will be. There are different concentrations of production and the effect of monitoring the concentration of the material on the size of the produced nanoparticles . By changing the size concentration of nanoparticles to nanometer has decreased. In the case of synthesis of nanoparticles and increasing the size of the produced particles, it increases from nanometer to micro.
Conclusion :
The influence of the monitoring amount of the substance in the synthesis of silver nanoparticles has been made, which has increased the resulting particles by increasing the average size of the resulting particles from nanometers. Synthesized nanoparticles in different concentrations that increase the monitoring concentration of the substance, there is no change in the identification of the nanoparticles, and the resulting nanoparticles are quasi-spherical. But with the increase of the average monitoring value, the particle size increases from nanometer to micro . On the other hand, increasing the concentration of the precursor increases the size of the particles; The resulting nanoparticles face an increase in volume.
Researcher and author: Dr. ( Afshin Rashid)
Specialized doctorate in nano-microelectronics