- Section (endohistal nano-fullers)
Investigation of endohistal fullerene c 320 nanofullers
Researcher and author: Dr. ( Afshin Rashid)
_yezi.gif)
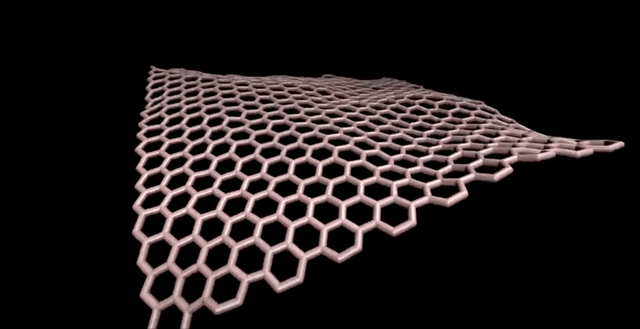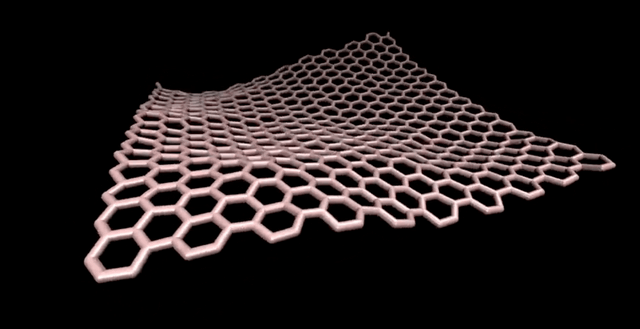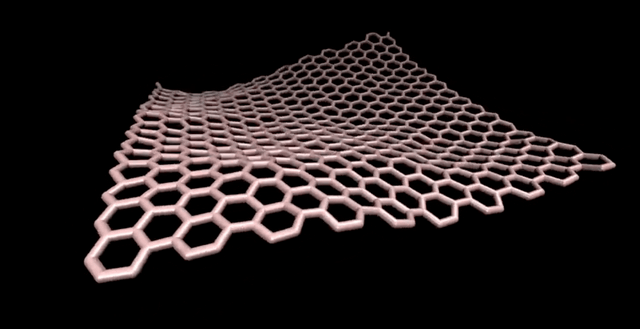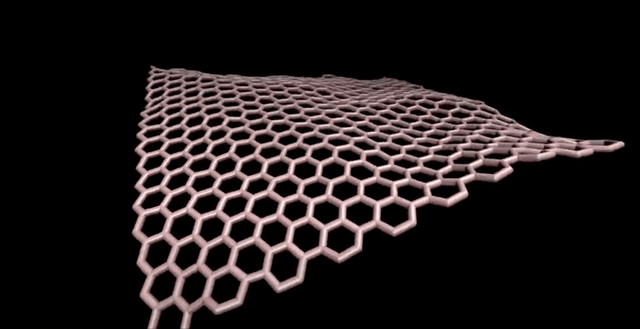
Note: There is the importance of using computational methods in exploring different sizes of C320 fullerene and their isomers.
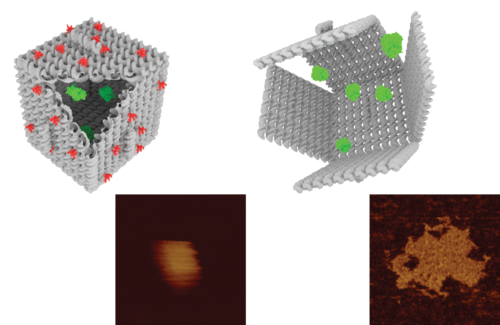
Fullerenes are nanometer-sized molecules that, in their simplest form, form 320 carbon atoms of a graphite layer with a three-dimensional, curved structure. 320 Unlike diamond and graphite, whose molecules are continuous, fullerenes have closed molecules: like c320 and ... (320) fullerenes, which are also called buckyball and buckytube, include nanotubes, nanofibers, fullerene has a structure similar to graphite, but instead of completely hexagonal sections, carbon atoms are placed in the vertices of the 5th or 7th polygons.
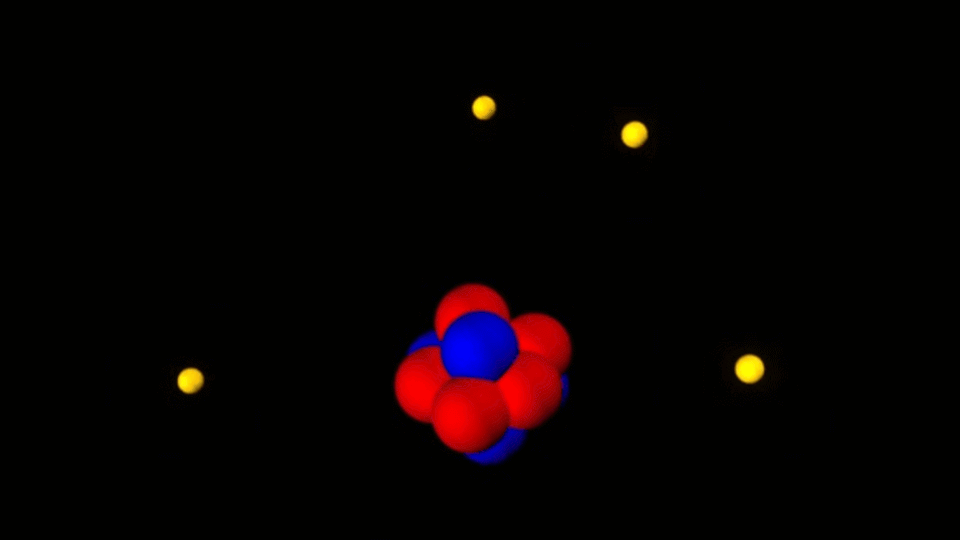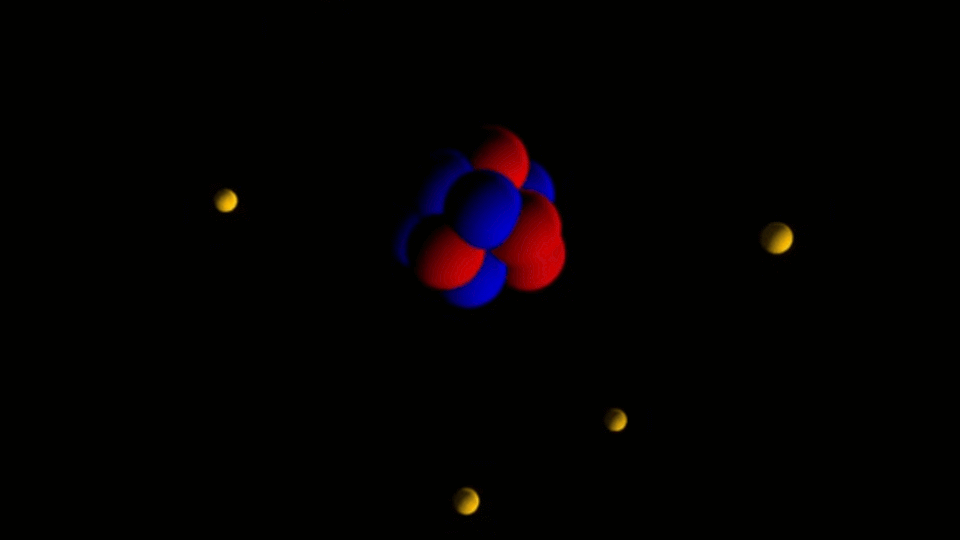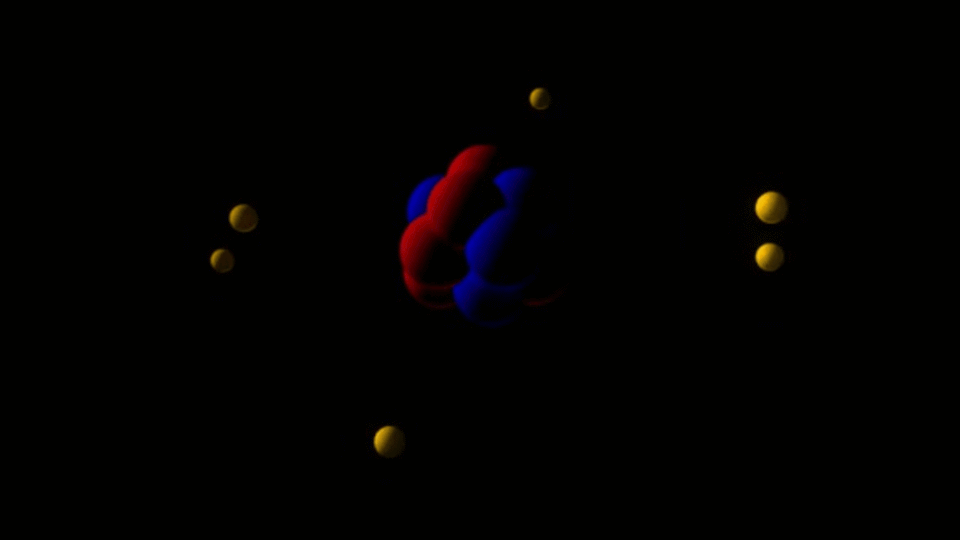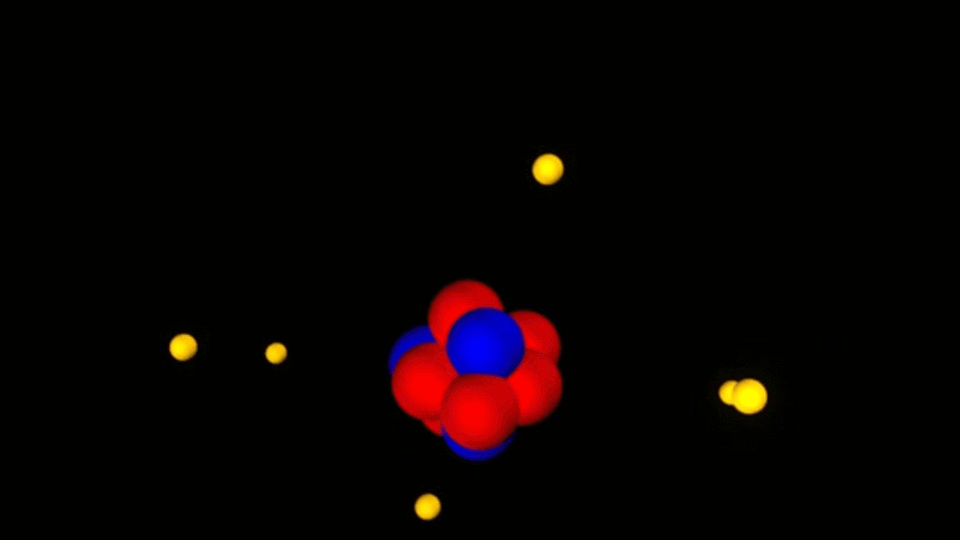
The important feature of all nanostructures is that the number of surface atoms in them is more than the number of volume atoms. This ratio increases with decreasing nanoparticle size. Therefore, the size of the nanoparticle is considered its most important feature. The range of nanoparticle activity change depends on the nature and shape of the nanostructure. However , if the energy of the nanoparticle field is comparable to the energy of electromagnetic radiation and if significant changes are made in a certain wavelength range with the occurrence of chemical reactions in the irradiated materials, the activity of nanoparticles up to 100nm will be significant.
_nph3.gif)
The surface atoms of nanoparticles are not compensated in terms of energy. In general, the results of nanoparticle energy growth can be expressed as the total energy of atoms on the surface of the particle. The freedom of movement of atoms on the surface of nanostructures is limited and only vibrational movements and the movement of electrons are possible. These two electrokinetic reactions are dependent on each other because the displacement of the electron clouds of the atoms definitely changes the vibrational frequencies of the bonds of the atoms of the nanoparticles . On the other hand, changing the location of valence electrons in bonds changes the polarity of bonds and bodies called supermolecules . In this case, electron transfer to a higher energy level becomes possible.
Conclusion :
Fullerenes are nanometer-sized molecules that, in their simplest form, form 320 carbon atoms of a graphite layer with a three-dimensional, curved structure. 320 Unlike diamond and graphite, whose molecules are continuous, fullerenes have closed molecules: like c320 and ...
Researcher and author: Dr. ( Afshin Rashid)
Specialized doctorate in nano-microelectronics